Abstract
Background
Whole-body low-dose multidetector computed tomography (MDCT) is used to assess bone lesions of multiple myeloma (MM), because it can provide more information on lytic bone lesions than plain conventional X-ray examination. MDCT can also depict bone marrow involvement of the appendicular skeleton (AS) in patients with MM. However, there is little evidence whether MDCT-based imaging can predict prognosis in patients with MM. Previously, we reported the prognostic implications of medullary abnormalities of AS in patients with MM (Blood Cancer J 2015;5:e2329). Here, we updated our previous observations and confirmed the prognostic relevance of medullary abnormalities of AS detected by MDCT in patients with MM.
Patients and Methods
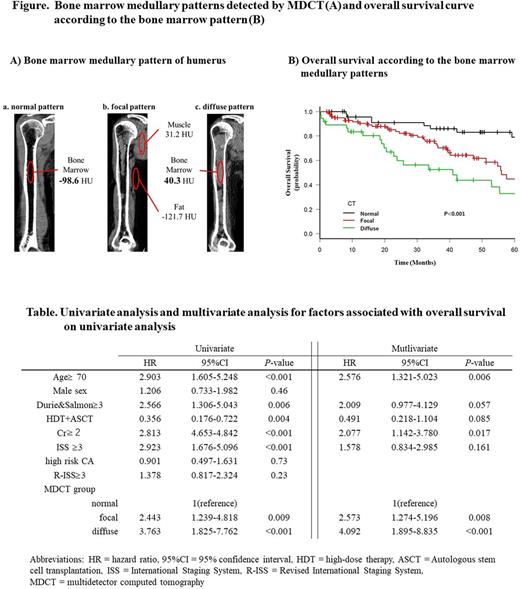
The initial study population consisted of 193 consecutive patients with newly diagnosed MM (NDMM) between January 2008 and June 2017 at Kameda Medical Center, Japan, undergoing MDCT in parallel with initial MM work-up. Six patients with cardiac amyloidosis were excluded because of its impact on survival. To differentiate marrow infiltration from physiological residual hematopoietic red marrow, a threshold of 30 HU was set. The normal pattern was defined as an absence of focal or diffuse high-density area in the medullary lesion with ≥ -30 HU recognized by MDCT. The diffuse pattern was defined as the presence of the abnormal CT value accounting for ≥ 70% of the bony canal. The focal pattern was defined as the presence of circumscribed high-density area which did not meet the definition of the normal or diffuse pattern (Figure A). Statistical analyses were performed with EZR, which is a graphical user interface for R ver. 3.2.2
Ethical considerations
This study was approved by the institutional review board of Kameda Medical Center and conducted in accordance with the principles of the Declaration of Helsinki.
Results
The median age of the patients was 73 years and median observation period was 34.3 months. Among 187 patients, the numbers of patients with normal, focal, and diffuse pattern were 49 (26%), 101 (54%), and 37 (20%), respectively. All of the patients were treated with bortezomib or immunomodulatory drug (IMiD)-based combined chemotherapy in the frontline setting. There were no significant differences in age, sex, M-protein subtype, or clinical response between the three groups. There were significant differences in the proportion of patients with International Staging System (ISS) stage III (33%, 55%, 81%, respectively; P < 0.001) and Revised International Staging System (R-ISS) stage III (14%, 27%, 49%, respectively; P = 0.009) between the normal, focal, and diffuse medullary patterns of AS. The differences in progression free survival (PFS) between the three groups were not statistically significant (log-rank test P=0.18), but overall survival (OS) showed significant differences between groups (log-rank test P < 0.001; Figure B). The 5-year survival rates were 79%, 44%, and 33% for normal, focal, and diffuse patterns, respectively. Median OS values were 92 months, 56 months, and 41 months, respectively. The mortality risk was associated with age, ISS III, Druie & Salmon stage III, creatinine ≥ 2 mg/dL, and receipt of autologous stem cell transplantation (ASCT) on univariate analysis. On multivariate Cox regression analysis, the presence of abnormal medullary lesions detected by MDCT remained as the independent prognostic predictor for OS (normal vs. focal; hazard ratio 2.573; 95% confidence interval, 1.274 - 5.196; P= 0.008: normal vs. diffuse; hazard ratio 4.092; 95% confidence interval, 1.895 - 8.835; P < 0.001: Table).
Conclusion
Our results indicated that the presence of abnormal bone marrow lesions in the AS detected by MDCT is an independent prognostic factor in patients with NDMM, and patients with a diffuse pattern have shorter survival period than those with the focal pattern. Therefore, assessment of bone marrow involvement of AS detected by MDCT should be considered not only for diagnostic purposes for bony lytic lesions but also to predict the prognosis of NDMM in addition to other imaging modalities.
Kitadate: Otsuka: Research Funding; Fujimoto: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Toyama kagaku: Research Funding; Chugai: Research Funding; Asahi Kasei: Research Funding; Kyowa Kirin: Research Funding; Eisai: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal